Deputy Director of the Drug Administration of Vietnam (DAV), under the Ministry of Health, has confirmed that the improper disposal of pharmaceuticals by Central Pharmaceutical Joint Stock Company No. 3 (CP3) at a landfill in Da Nang constitutes a violation of legal procedures for drug destruction.
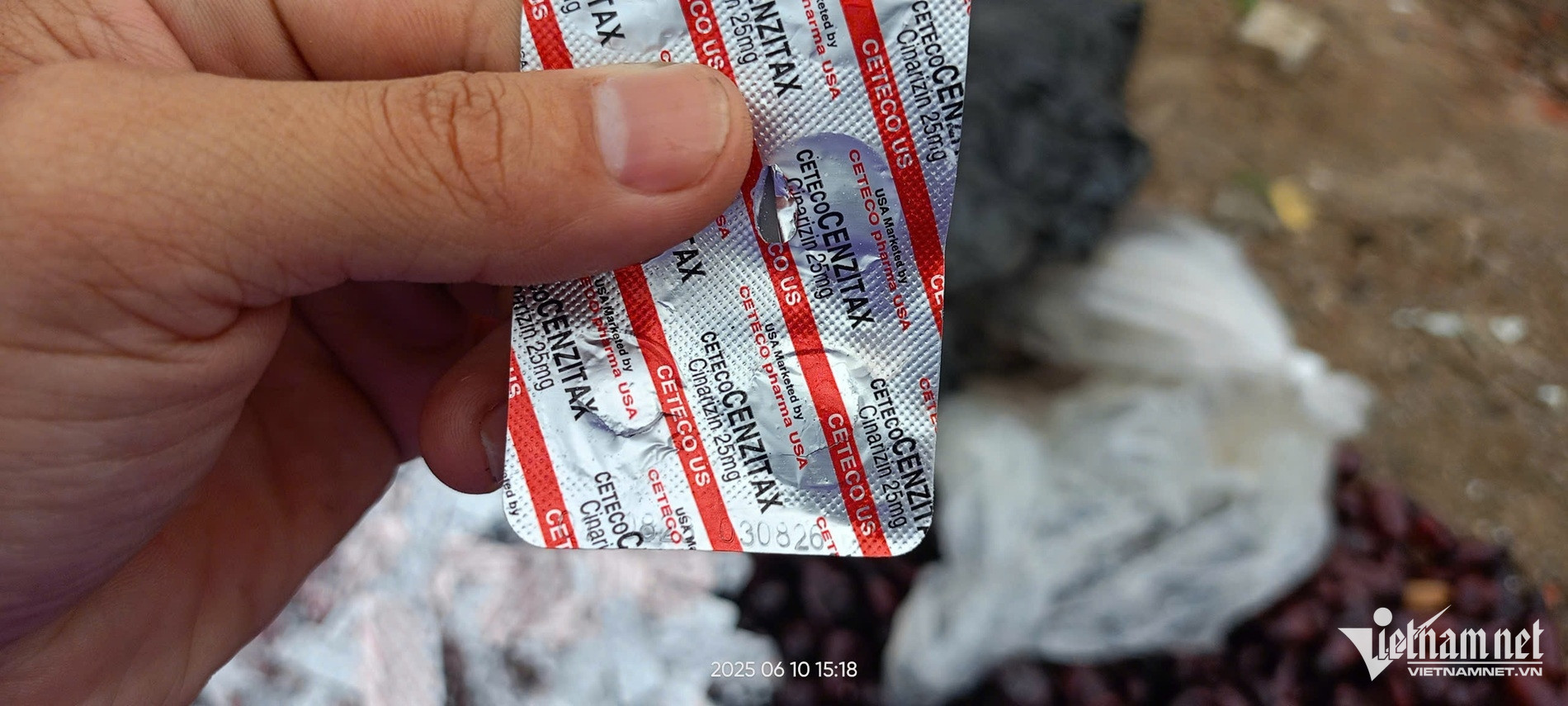
The statement was made following public reports that large quantities of the drug Cetecocenzitax 25mg - clearly marked with an expiry date of August 2026 - were found dumped along Xuan Thieu 21 Road in Da Nang. The medication is manufactured by CP3.
Speaking with the press on June 13, Ta Manh Hung, Deputy Director of DAV, stated that the disposal of pharmaceuticals must follow the regulations outlined in Circular No. 11/2018 by the Ministry of Health. According to the circular, the head of any facility possessing drugs for disposal must form a disposal council with at least three members, including one responsible for professional oversight.
Additionally, drug disposal must ensure safety for humans and animals and must avoid environmental pollution in accordance with legal guidelines.
The responsible facility bears full accountability for the drug disposal process and must submit a report, including the minutes of the destruction, to the local Department of Health. In this case, CP3 is required to report to the Da Nang Department of Health.
After discussions with police, CP3 retrieved all the improperly discarded drugs and is proceeding with proper destruction measures.
Previously, on September 6, 2024, the DAV issued an official letter mandating the recall of Cetecocenzitax tablets (Cinarizine 25mg) produced by CP3 due to substandard quality. This medication is typically used to prevent motion sickness and alleviate symptoms such as dizziness, tinnitus, nausea, and vomiting associated with vestibular disorders.
A source from CP3 confirmed the drugs found discarded belonged to the company and had been recalled for destruction since September 2024. The individual explained that because the blister packaging was made of aluminum, scrap collectors may have picked them up from a landfill, hoping to resell them. Unable to do so, they may have abandoned the packages along Xuan Thieu 21 Road.
When must recalled drugs be destroyed under current regulations?
Clause 2, Article 15 of Circular No. 11/2018/TT-BYT (as amended by Clause 12, Article 1 of Circular No. 03/2020/TT-BYT) outlines the following cases in which recalled drugs must be destroyed:
Drugs recalled due to violations classified as Level 1 or Level 2.
Drugs recalled for Level 3 violations, where the Ministry of Health (DAV) determines they cannot be remediated or re-exported.
Drugs recalled for Level 3 violations that the Ministry of Health (DAV) has allowed to be remediated or re-exported, but the facility is unable to do so.
Counterfeit drugs, smuggled drugs, drugs of unknown origin, expired drugs, drugs containing banned substances, drugs produced from substandard raw materials, and drugs required to be destroyed under administrative penalties in the healthcare sector or expired retention samples.
Vo Thu